List of terms
Terms commonly encountered when talking about environmental radioactivity and radiation are explained here.
A
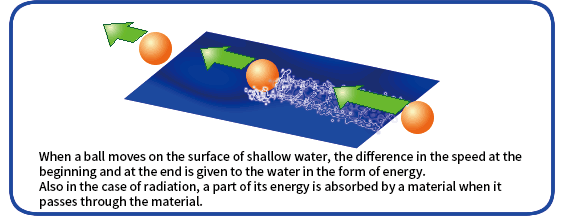
When radiation passes through a material, some of the energy of the radiation is absorbed by the material.
How energy is transferred depends on the type of the radiation. Absorbed dose (J/kg) is energy of radiation (J) absorbed by a unit mass (kg) of a material. Generally, however, absorbed dose is expressed in gray (Gy). 1 Gy is 1 J/kg.
(Reference: Atomic Energy Encyclopedia ATOMICA)
A chemical analysis method utilizing absorption of light by materials. When a light passes through a solution, the degree of absorption of the light is governed by the concentration of materials in the solution. Using this nature, the concentration of the materials can be determined.
Airborne dust and mist. Measuring the radioactivity of airborne dust samples enables estimating the amount of radioactivity taken from the air into the human body through breathing.
Dose of γ-rays in the air around nuclear facilities or general environment. One of measurement items of radiation monitoring. It mainly comes from γ-rays emitted from natural radionuclides contained in the ground or buildings (includes cosmic rays). Dose that originates from charges generated in the air by radiation is called exposure dose (in μR/h, etc.), and dose that originates from energy absorbed in the air is called air absorbed dose (in nGy/h, etc.). Dose rates can also be obtained by converting count rates.
Particle radiation released from nucleus, which actually is the high-energy nucleus of helium (He-4). Energy of α ray is determined by the releasing nuclide, in the range of 4-8 MeV. Energy of α ray dissipates quickly in a substance, and therefore the range (distance radiation can travel) is extremely short. The range of 5-MeV α ray in the air is around 3.8 cm.
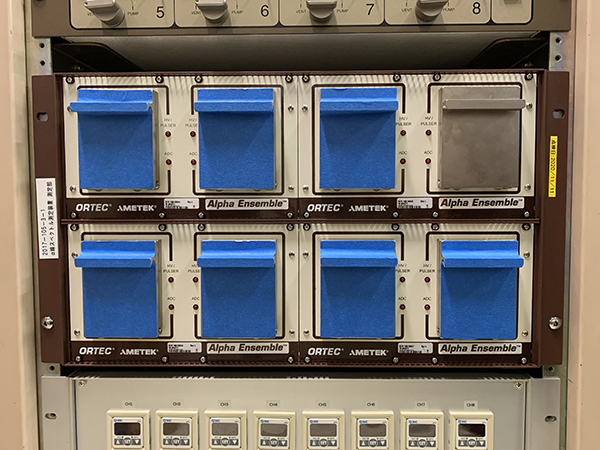
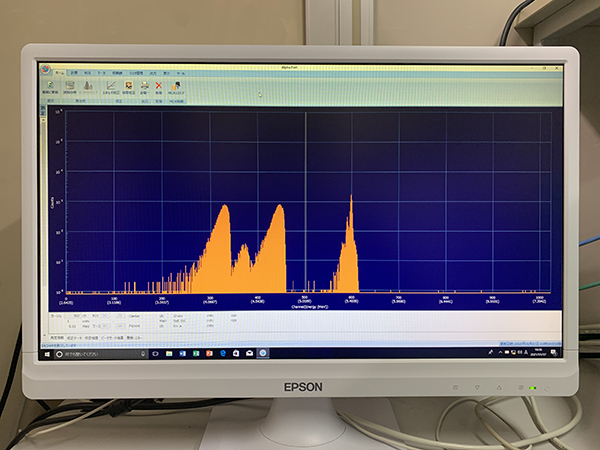
The technology to measure α-ray spectra and the technique to analyze the spectra to determine the radioactivity. Currently, silicon semiconductor detectors are used primarily, and almost all of α-emitting nuclides in environmental samples are measured by this method.
Silicon semiconductor detector
Alpha-ray spectrum
Radionuclides that are artificially created through nuclear tests or in nuclear reactors, including cobalt-60, strontium-90, cesium-137, and plutonium.
Nuclear tests that are performed in the atmosphere. Compared with underground nuclear tests, atmospheric nuclear tests cause radioactivity to spread globally and fall onto the ground along with rainfall. The last atmospheric nuclear test in the world was the 26th Chinese nuclear test conducted in October 1980.
Atomic number equals the number of protons in the nucleus. The type of element is determined by the atomic number (symbol: Z). For example, Z = 1 is hydrogen (H), and Z = 6 is carbon (C).
B
Counts or spectra obtained without setting a sample to or by setting a blank sample free of radioactivity to radiation measurement equipment are called background.
The becquerel is the SI derived unit of radioactivity. One becquerel is defined as the quantity of radioactive material in which one nucleus decays per second on average. This unit is named after A. H. Becquerel, who received a Nobel Prize in Physics in 1903 for discovering radioactivity of uranium.
Fast electrons discharged from nucleus are called β-rays. There are negatively charged β-rays and positively charged β-rays (called positive electron or positron). Energy of a β-ray is a continuous distribution from zero to the maximum energy, and is usually expressed by the maximum energy.
C
According to JIS Z 8103:2000, calibration is defined as “set of operations that establish the relationship between values of quantities indicated by a measuring instrument or measuring system, or values represented by a material measure or a reference material, and the corresponding values realized by standards. Note: calibration does not include correction of errors by adjusting measuring instrument.”
Carbon-14 (C-14) decays with releasing β-rays. Its half-life is 5,730 years. Carbon-14 found in the environment is created by nuclear reaction between neutrons generated by cosmic rays and nitrogen nuclei in the atmosphere. In the atmosphere, the generation rate of carbon-14 equals the decay rate of carbon-14 and the ratio of carbon-14 to the stable carbon-12, 14C/12C, remains almost constant. Animals and plants take in carbon at this ratio, and the 14C/12C ratio in their bodies is the same as the 14C/12C ratio in the atmosphere. When they die, carbon-14 decays with the half-life of 5,730 years, and the 14C/12C ratio starts to change. Using that, we can date diseased animals and plants. This is known as radiocarbon dating.
A radioactive isotope with a half-life of 30 years that is generated by nuclear fission of uranium and other nuclides. Its chemical nature is very close to potassium, and therefore it tends to accumulate in muscles when taken into our bodies. Cesium-137 that is found in nature mostly comes from atmospheric nuclear tests, but the current concentration of cesium-137 in rainwater and dust has dropped to about 1/20 of the concentration in the 1970s.
A chemical separation method utilizing a highly selective resin that strongly adsorbs certain ions through chelation. The term chelate comes from the Greek word chela, meaning “crab’s claw.”
A nuclear accident occurred in the Ukrainian Soviet Socialist Republic on April 26, 1986. A reactor at the power plant exploded and caught fire, which caused a large amount of radioactivity to spread globally.
When cobalt-59, a stable nucleus, absorbs a neutron in a reactor, it becomes cobalt-60 by nuclear reaction called neutron capture. Cobalt-60 has a half-life of 5.3 years, and decays with emitting β-rays. In so doing, cobalt-60 releases 1173- and 1332-keV γ-rays. It is an important nuclide for monitoring nuclear facilities. Hardly any cobalt-60 exists in the environment now.
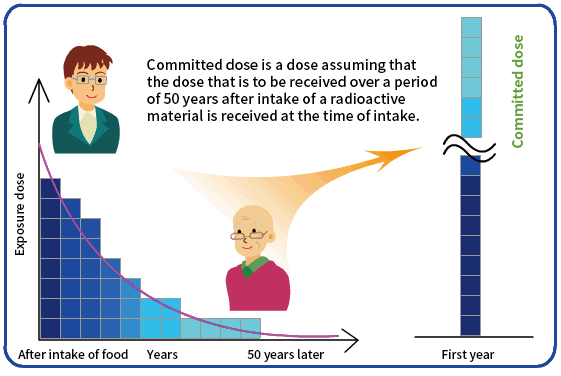
After a radioactive material is taken into a human body, its radioactivity decays in accordance with its half-life. Also, it will be gradually discharged out of the body through metabolism. While that is all happening, the tissues and organs in the body continue to be exposed to radiation emitted from the radioactive material.
Committed dose is a radiation dose assuming that the dose that is to be received over a committed period of 50 years (for adults) or 70 years (for children and infants) after intake is received at the time of intake.
(Reference: Atomic Energy Encyclopedia ATOMICA)
The CTBT is a treaty that bans nuclear weapon tests anywhere in the atmospheric, extra-atmospheric (including space), underwater, and underground environments, which was adopted by the United Nations General Assembly in September 1996. The CTBT was epoch-making since the Partial Test Ban Treaty (PTBT) prepared in 1963 did not ban underground nuclear tests. The CTBT has not entered into force, as it requires ratification by the designated 44 states including the five recognized nuclear-weapon states and India, Pakistan, and Israel. The 1st Conference on Facilitating the Entry into force of the CTBT was held in October 1999, and the 2nd Conference in November 2001. The CTBT plans to establish the Comprehensive Nuclear Test-Ban Treaty Organization (CTBTO) for the effective implementation of the CTBT, and stipulates international monitoring systems. The United Kingdom, France, and Russia have ratified the CTBT. However, the US Senate rejected ratification in October 1999. The Bush administration was absent from the 2nd Conference in November 2001, and was criticized for trying to make the CTBT a dead letter.
(Reference: Atomic Energy Encyclopedia ATOMICA)
The primary cosmic rays that come from space are extremely high-energy protons. The primary cosmic rays collide with atoms such as nitrogen in the atmosphere and generate various kinds of secondary cosmic rays (e.g., electrons, γ-rays, X-rays, mesons, neutrons).
Conversion coefficient to determine radioactivity from measured count rates. It essentially means the rate of radiation counts for the measurement target. In the case of spectrum measurement, the counting efficiency with respect to the net peak count rate (peak efficiency) is used.
Measurement equipment that counts the amount of radiation detected expresses the results in count rates, counts divided by the measuring time. Customarily, count rates are expressed in cps (counts per second) or cpm (counts per minute).
D
Decontaminate it to remove radioactive materials or cover them with soil to reduce the amount of radiation we receive in places we live.
■ Remove
Radioactive materials are removed from inhabited areas by scraping off the surface soil, removing branches and fallen leaves, and washing the surface of buildings to which radioactive materials are attached.
■ Shield
Radiation from radioactive materials can be shielded by covering them with soil or concrete, resulting in lower air radiation doses and exposure doses.
■ Keep away
The strength of radiation decreases as the distance from radioactive materials increases. Therefore, keeping radioactive materials away helps with reducing exposure doses of people. Shortening the time of being near a radioactive material also essentially means “keeping away” from it.
(Reference: Environmental Remediation website of the Ministry of the Environment) * The image is taken from the Environmental Remediation website of the Ministry of the Environment

Ddose
It refers to the amount of radiation. Commonly the following three types of “dose” are used.
1) Exposure dose: dose that is defined by the charges generated in the air, usually expressed in C/kg or roentgen (R).
2) Absorbed dose: dose that is defined by the amount of energy of radiation absorbed by a material, usually expressed in gray (Gy).
3) Dose equivalent: dose that takes into account the biological impacts of radiation on the human body, usually expressed in sievert (Sv).
Equipment for measuring dose or dose rate. Dosimeters are available in various types, including stationary types that are installed at a specific place for measurement, portable-type dose measurement equipment (survey meters), film badges for measuring doses for a long period of time, and thermoluminescent dosimeters (TLDs). Commonly used detectors for the former two are ionization chambers and NaI(Tl) scintillation detectors.
In Japanese, this is called the kagezen method. Kagezen is a practice in Japan where an extra portion of meal is prepared and set out for a person who is on a trip away from the family, as if the person is present at the table, to pray for the person’s safety and health. A method to assess dietary intake by retaining a duplicate portion of all food and drinks consumed. It is one of the methods for sampling daily food. (Reference: The Series of Environmental Radioactivity Measuring Methods 16 Method for Sampling of Environmental Materials)
E
The effects of radiation on the human body vary depending on the type and nature of the radiation and the type of tissue or organ of the human body. Effective doses are radiation doses that take into account these factors, and are used in radiation exposure controls.
The effective dose used in exposure control is derived by calculating [Absorbed dose] × [Radiation weighting factor] × [Tissue weighting factor] for each of tissues and organs and summing the products for all the tissues and organs.
(Reference: Atomic Energy Encyclopedia ATOMICA)
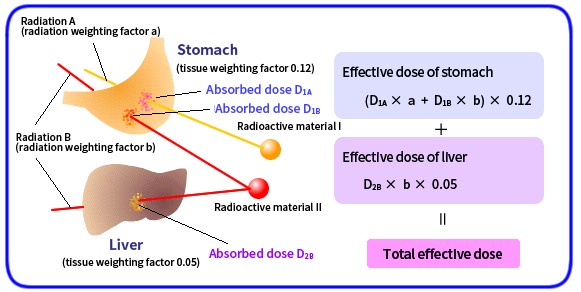
It is not easy to calculate the dose a tissue or organ receives from a radioactive material taken into the body, because that requires measuring the amount of the radioactive material remaining in the tissues and organs and monitoring the temporal change of the amount.
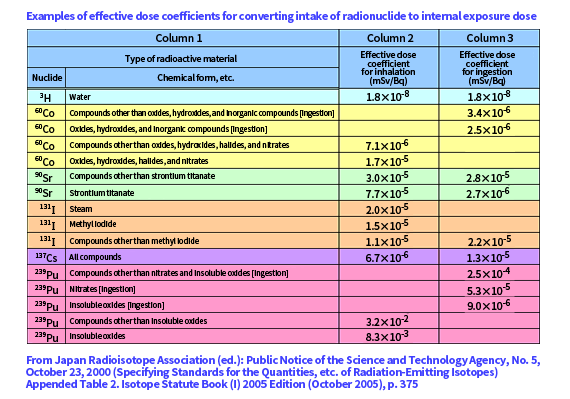
To address that, the relationship between the amount of a radioactive material taken in and the dose a tissue or organ receives is predetermined to calculate the exposure dose corresponding to the amount of the radioactive material. The coefficient to express the relationship between the amount of a radioactive material taken in and the exposure dose is called effective dose coefficient.
(Reference: Atomic Energy Encyclopedia ATOMICA)
When two electrodes, one being a platinum wire and the other being a copper plate for example, are set in a solution and direct current voltage is applied to the electrodes, metal ions in the solution are deposited onto the copper plate. This process is called electrodeposition, or electroplating.
Environmental radiation monitoring conducted in the case of abnormal release of, or possible abnormal release of, radioactive materials or radiation.
(Reference: Nuclear Emergency Response Guidelines)
Energy of radiation is expressed in electron volt (eV). This is defined as energy an electron gains when accelerated by a potential difference of 1 V, but it is used to express energy for all radiation types, including α-rays, β-rays, X-rays, γ-rays, and neutron rays. Generally, 1,000 times of eV (keV) or 1,000,000 times of eV (MeV) is used as the unit.
It is an indication of precision of measurement of energy of α- or γ-rays, where peak spreading is expressed in % or keV. The energy resolution of NaI(Tl) scintillation detectors is not very good with 5% to 15%. Meanwhile, germanium semiconductor detectors have an excellent energy resolution of 1% or less.
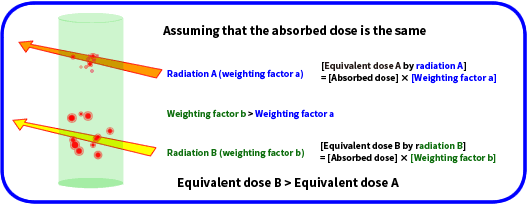
The influence of radiation on a human body when it passes through the human body requires considerations to the differences that arise from the type of the radiation, not just the energy the radiation gives to the human body. How radiation gives energy to a human body depends on the type of the radiation.
The degree of influence on a human body can be derived by multiplying the amount of energy given to the human body (absorbed dose) by a factor that takes into account the differences that arise from the type of radiation (called radiation weighting factor).
The calculation formula is:
[Equivalent dose] = [Absorbed dose] × [Radiation weighting factor]
(Reference: Atomic Energy Encyclopedia ATOMICA)
In nuclear science, the term “exposure” usually refers to exposure of a human body to radiation, and the amount of radiation the human body was exposed to is called exposure dose.
In normal radiation exposure controls, equivalent doses (calculated by multiplying the absorbed dose of the human body (Gy) by the radiation weighting factor determined for each radiation type) or effective doses (calculated by multiplying the equivalent dose by tissue weighting factors that take into account the radiosensitivity of tissues and organs and summing the products for all tissues and organs) are used, which are both expressed in sievert (Sv).
(Reference: Atomic Energy Encyclopedia ATOMICA)
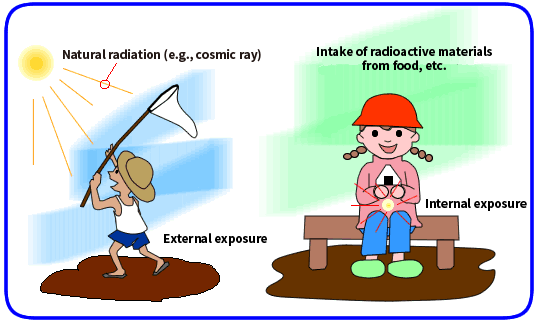
When radioactive materials or radiation sources are present outside a human body and the human body is exposed to radiation coming from outside the body, it is called external exposure. When radioactive materials are present inside a human body and the human body is exposed to radiation coming from inside the body, it is called internal exposure.
External exposure is caused by natural radiation such as cosmic rays, and internal exposure occurs by radioactive materials being taken into our bodies through inhalation of air or ingestion of food and drinks that contain radioactive materials. A large majority of internal exposure is attributable to radioactive materials of natural origin.
(Reference: Atomic Energy Encyclopedia ATOMICA)
F
Artificial radioactive materials generated by atmospheric nuclear tests and other means may disperse in the atmosphere and fall onto the ground along with dust. Such radioactive materials are called radioactive fallout. Representative radioactive fallouts are strontium-90 and cesium-137. It’s been so long since the last atmospheric nuclear test was conducted, and the amount of radioactive fallout has become substantially less compared to the amount of natural radioactivity.
Fuming nitric acid is a concentrated nitric acid solution containing nitrogen dioxides. Strontium nitrate barely dissolves into fuming nitric acid, and using this property strontium can be chemically separated from other elements, including its homologous element calcium.
G
One type of electromagnetic wave (same as radio waves, light, and X-rays) released from nuclei, and the energy of γ-ray is specific to the releasing nuclide. Therefore, the releasing nuclide can be determined by accurately measuring the energy of γ-ray by γ-ray spectrometry using germanium semiconductor detector. For example, the energy of γ-rays released from cesium-137 is 662 keV, while that of γ-ray released from cobalt-60 is 1173 and 1332 keV.
The technology to measure γ-ray spectra and the technique to analyze the spectra to determine the radioactivity. Currently, germanium semiconductor detectors are used primarily, and almost all of γ-emitting nuclides in environmental samples are measured by this method.
A highly pure germanium crystal cooled to the temperature of liquid nitrogen (–196°C) functions as a sensor to convert energy of radiation to electric signals. For their extremely high energy resolution, germanium semiconductor detectors are widely used in the measurement of γ-ray spectra of environmental samples, used in combination with multichannel pulse height analyzers. The detector part is shielded by a thick wall of lead (10 cm or thicker) to reduce background radiation.

Seiko EG&G product

Mirion Technologies Canberra product
H
A half-life is the time required for radioactivity to become half of its initial value. A half-life is fixed for the nuclide. Some nuclides have a half-life much shorter than 1 second, and some nuclides have a half-life longer than several billion years.
Hormesis refers to a phenomenon where a certain material is detrimental when used in high concentrations or in larger doses, yet it is beneficial when used in low concentrations or in smaller doses.
(Reference: Hormesis Clinical Study Group website)
I
Iodine-131 is generated by nuclear fission of uranium-235. It’s an artificial β- and γ-emitting radionuclide with a half-life of 8 days. For its high volatility, iodine is released into the environment the most after a nuclear test or accident. For that reason, iodine-131 is one of the important nuclides in environmental radiation monitoring.
A chemical separation method using an insoluble synthetic resin that has ion exchange groups. It utilizes the nature of metal ions in a sample solution to strongly adsorb to or desorb from the ion exchange resin depending on the chemical characteristic of the solution.

Ion exchange resin method
It is a type of radiation detector that measures ion pairs (electrons and cations) created in the air through ionization by radiation. It is used for the identification and dose measurement of gaseous radionuclides (e.g., krypton-85).
Ionizing radiation is radiation that ionizes materials, which is simply expressed as “radiation.”
Ionizing radiation includes directly ionizing radiation, such as charged particles (e.g., alpha-rays and beta-rays), that directly ionizes atoms and molecules and indirectly ionizing radiation, such as X-rays and neutron rays, that generates charged particle rays through interaction with the bound electron or nucleus of atom and the generated charged particle rays ionize materials.
(Reference: Atomic Energy Encyclopedia ATOMICA)
Nuclides of the same element (same atomic number) with different mass numbers are called isotopes. Radioactive isotopes are called radioisotopes. For example, cesium-134 and cesium-137 are radioisotopes of cesium.
L
Scintillator using a liquid made by dissolving organic fluorescent substances in toluene or xylene. In the case where the measurement sample is water, an emulsion scintillator in which a surfactant is added to the scintillator is used to allow mixing with (gelling) the water up to 40-50%.
Equipment for measuring radioactive isotopes that emit β-rays. Since the radioactivity concentration in general environmental samples is very low, a thick shielding substance (e.g., lead) is employed to reduce the influence of radiation from external sources.

low-background beta counter
Liquid scintillation counters that use special equipment or methods to reduce background count rates. They are used for the measurement of nuclides that exist in the environment in very small amounts, such as tritium (H-3) or carbon-14 (C-14).

low-background liquid scintillation counter
M
One of the theoretical living expense calculation methods. In the market basket method, a standard quantity is derived for each necessary item in life, such as food and medical services, which is multiplied by the price, and the products are summed for all the necessary items to get the picture of people’s lives. It is often used for calculating the minimum cost of living, and for preparing material for requesting a pay rise for laborers.
(Reference: weblio online dictionary (Daijirin, Sanshodo))
Mass number (A) is the sum of the number of protons (Z) and the number of neutrons (N) contained in a nucleus, i.e., A = Z + N.
A method for analyzing materials by ionizing the material and distinguishing the differences in the mass of atoms and molecules utilizing the interaction with electromagnetic fields. (Reference: Encyclopedia Nipponica)

mass spectrometry
It refers to an operation to repeatedly separate or extract a short-lived daughter nuclide from a parent nuclide with a long half-life while they are in radioactive equilibrium. The term comes from its similarity to milking dairy cattle after a certain interval.
(Reference: Atomic Energy Encyclopedia ATOMICA)
Continual measurement and monitoring of radiation doses or the concentration of radioactive materials in nuclear facilities or the environment is called radiation monitoring. Environmental radiation monitoring includes radiation (primarily air γ-ray dose) in the environment and radioactivity monitoring of environmental samples.
A field radiation measurement point equipped with air radiation measurement equipment such as NaI(Tl) scintillation detector for the purpose of measuring and monitoring outdoor air radiation levels. Measurement equipment is installed outdoors (e.g., building rooftop), and the measurement data are sent to a central monitoring device to monitor them.

General view

Sensor part
N
NaI(Tl) is an inorganic crystalline fluorescent substance (scintillator). It is highly sensitive in detecting γ-rays, and able to measure the energy. However, the energy resolution is not very high.
Stationary γ-ray measurement equipment that uses NaI(Tl) scintillation detector. It measures γ-rays inside radiation facilities and in the general environment, and it can display both count rates and dose rates.
Portable radiation measurement equipment that uses NaI(Tl) scintillation detector. It is primarily used in field measurement of γ-rays, and it can display both count rates and dose rates.

NaI(Tl) scintillation survey meter
Natural radiation is mainly radiation from natural radionuclides (potassium-40, uranium series nuclides, and thorium series nuclides) that exist in the soil, cosmic rays, and radiation from nuclides created by cosmic rays colliding with atoms in the air.
Natural radionuclides include radionuclides that exist on the Earth since its creation (e.g., potassium-40, uranium series nuclides, thorium series nuclides) and nuclides that are generated by collision of cosmic rays and atoms in the atmosphere (e.g., tritium, carbon-14).
Uncharged neutral elementary particle with a mass almost the same as protons. Neutrons generated by nuclear fission or nuclear reaction are fast neutrons, but they decelerate and lose energy when they pass a material that contains many hydrogens (moderator) such as water. Eventually, the fast neutrons become thermal neutrons with almost zero energy.
When a stable nucleus is placed in a reactor and a neutron is irradiated on it, the nucleus changes to a one mass number larger nucleus through nuclear reaction called neutron capture. If the newly created nucleus is a radionuclide, measuring the radioactivity of the activated sample by γ-ray spectrometry enables us to determine the type (element) and quantity of the original stable nucleus. Neutron activation analysis can quantity trace amount elements at ppb (part per billion) levels.
Guidelines to facilitate smooth implementation of nuclear emergency measures by nuclear operators, heads of designated administrative organs, heads of designated local administrative organs, local governments, designated public institutions, designated local public institutions, and other persons pursuant to Article 6-2, paragraph 1 of the Act on Special Measures Concerning Nuclear Emergency Preparedness (Act No. 156 of 1999).
The purpose of the Guidelines is to ensure protective measures to minimize the impact of radiation on residents near nuclear facilities in emergencies, from the viewpoint that securing the life and safety of the people takes the highest priority.
(Reference: Preamble <Objective and Purpose> of Nuclear Emergency Response Guidelines)
When uranium-235 or plutonium-239 absorbs neutrons, it splits into two other nuclei and releases about 210 MeV of energy. Nuclides created by nuclear fission are called nuclear fission products or nuclear fission fragments.
Nuclide created by nuclear fission. Also called nuclear fission fragment. A majority of nuclear fission products are β- or γ-emitting radionuclides.
Ship in which a nuclear reactor is used for propulsion.
Nuclear powered warships can sail at high power and for a long distance on a small amount of fuel, and combustion of the nuclear fuel does not require oxygen. For that reason, a majority of currently existing nuclear powered warships are submarines.
For consumer use, Russia has many nuclear-powered icebreakers, including the world’s first nuclear-powered surface ship Lenin. Following the launching of Lenin, the merchant ship Savannah was built in the US, the coal carrier Otto Hahn in Germany, and the experimental ship Mutsu (experimental voyage of Mutsu was in 1991) in the 1960s. However, they all have been retired, and only several Russian icebreakers are operative now because nuclear-powered transport ships have little to no economic advantages under current conditions.
(Reference: ATOMICA)
It means the type of nucleus. All nuclides are determined by the atomic number and mass number. Nuclide symbols are expressed by the symbol for the element and the mass number. For example, the element with the atomic number of 55 is cesium, and the nucleus with the mass number of 137 is expressed as cesium-137 or 137Cs.
O
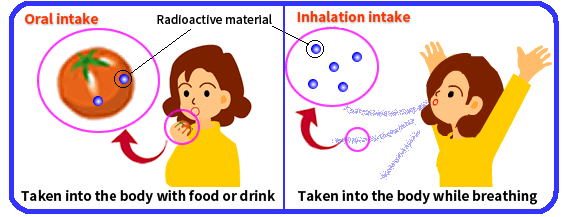
Oral intake is taking of radioactive materials into the body through ingestion of water, cereal, meat, milk, etc., that contains the radioactive materials.
Inhalation intake is taking of gaseous or particulate radioactive materials (minute particles with radioactive materials adsorbed onto; also called radioactive dust or radioactive aerosol) in the air into the lungs through breathing.
The radioactive materials are discharged out of our bodies through excretion or breathing, but some remain in our bodies.
(Reference: Atomic Energy Encyclopedia ATOMICA)
P
Plutonium-239, the representative nuclide of plutonium, is an artificial radionuclide created by uranium-238 absorbing neutron in a nuclear reactor. Plutonium-239 has a half-life of 24,000 years, and emits α-rays. It also absorbs neutrons and causes nuclear fission. Plutonium-239 is a very important nuclide in the monitoring of environmental radioactivity. After separating and refining, plutonium is measured using α-ray spectrometers.
Isotope of potassium contained in soil, etc. It takes up 0.0117% of natural potassium. Its half-life is 1.3 billion years, and it releases β- and γ-rays. It is one of the causes of background counts in γ-ray measurements.
R
Radiation comes in the form of electromagnetic radiation such as X-rays and γ-rays and particle radiation such as α-rays and β-rays. In either case, radiation is able to directly or indirectly ionize atoms in materials.
Radiation hormesis refers to a phenomenon that radiation, which is detrimental at higher doses, has beneficial effects at lower doses. The beneficial effects include improvement of biological activity and induction of adaptive response to develop resistance against high doses.
(Reference: Atomic Energy Encyclopedia ATOMICA)
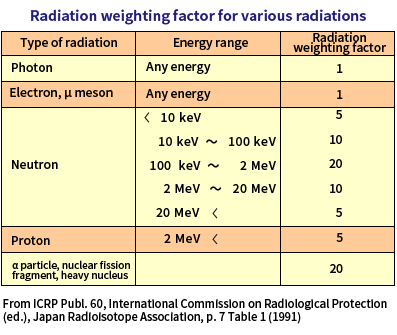
Even if the absorbed dose of radiation in the body is the same, its influence on the body varies depending on the nature of the radiation. The radiation weighting factor is a factor established to assess the influence of different radiation on our bodies by the same measure.
(Reference: Atomic Energy Encyclopedia ATOMICA)
The process in which a nucleus releases radiation and changes to another type of nucleus. Also called disintegration. Decay in which α-rays are emitted is called α-decay, decay in which β-rays are emitted is called β-decay, and decay in which an orbital electron is captured is called orbital electron capture decay. In general, the process of emission of γ-rays is not called decay since emitting γ-rays does not make the nucleus change its type.
It refers to a state where the ratio of the radioactivity of an radionuclide (parent nuclide) to that of a nuclide created by the decay of the said nuclide (daughter nuclide) remains the same for a certain period of time. Radioactive equilibrium includes secular equilibrium and transient equilibrium.
Almost identical to radionuclide in the meaning. Also called radioisotope.
The term radioactivity is used in the following two meanings:
1) the nature of nucleus to emit radiation and become another nucleus;
2) intensity of radioactive decay, expressed by the number of nucleus decays per second.
The unit of radioactivity is becquerel (Bq). When one nucleus decays per second on average, the radioactivity is 1 Bq.
An analysis method to separate and refine radionuclides in a sample and measure their radioactivity to determine the quantity of the radionuclides.
The same as radioactive isotope.
It refers to nuclide that emits radiation.
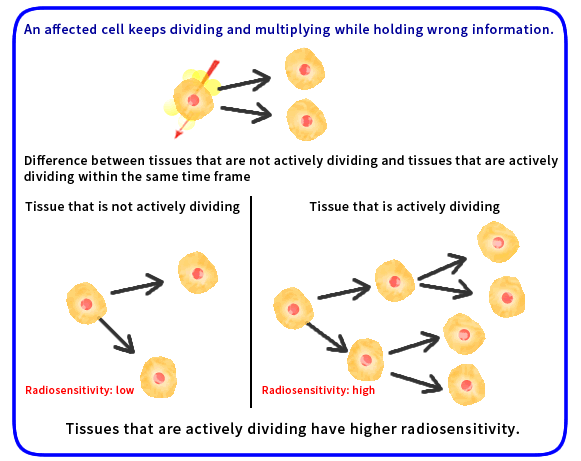
The influence of radiation on the body differs depending on the tissue or organ. This difference in the influence is called radiosensitivity. Influence of radiation on human bodies includes cancer or genetic effects caused by damage to DNA. Because DNA is used as a blueprint in cell division, actively dividing or multiplying tissues or undifferentiated cells (e.g., blood-forming tissues, reproductive glands, skins) are more susceptible and sensitive to radiation.
(Reference: Atomic Energy Encyclopedia ATOMICA)
Radium-226 was discovered by Marie Curie. Similar to uranium, radium is a radionuclide that is found in nature, such as in the soil. Radium-226 has a half-life of 1,600 years, and emits α-rays to become radon-222.
Gaseous radionuclide generated by decay of radium-226 that is often found in the ground. Although its half-life is short, 3.82 days, radon will be generated and disperses into the air as long as radium-226 exists in the ground. Since radon can be easily taken into our bodies through breathing and causes internal exposure (radon accounts for about half of the total exposure dose), radon is an important nuclide as environmental radioactivity.
For the purpose of this website, it refers to precipitations such as rain and snow as well as particles that fall along with them. Their radioactivity is measured to investigate the amount of radioactive fallout.
RAMISES, RAdiation Monitoring Information Sharing for Emergency Support, is a network system established to facilitate emergency monitoring. It aims to support prompt and appropriate gathering and sharing of radiation-related information (e.g., monitoring information, accident information) among parties related to disaster prevention and response entities (e.g., national and local governments).
(Reference: Nuclear Safety Technology Center website)
According to JIS Z 8103:2000, reference material is defined as “material or substance one or more of whose property values are sufficiently homogeneous and well established to be used for the calibration of an apparatus, the assessment of a measurement method, or for assigning values to materials. Note: may also be called standard sample.”
S
A fluorescent substance (scintillator) emits light when radiation hits it. A scintillation detector converts the light to electric signals to detect radiation. Various fluorescent substances are available to measure α-rays, β-rays, and γ-rays.
Mud or sand that were brought by river and deposited onto the seabed. Their radioactivity is measured to investigate the distribution of radioactivity accumulated on the seabed.
A highly pure silicon crystal functions as a sensor that converts radiation energy to electric signals. For their high energy resolution, silicon semiconductor detectors are used in α-ray spectra measurement, but they are also sometimes used in the measurement of β-rays and low-energy X-rays.

silicon semiconductor detector
The radioactivity of soil samples is measured to investigate the amount of radionuclides accumulated in the soil. Strontium-90 and cesium-137 fall onto the ground by rain, and slowly migrate within soil from the surface layer down to the lower layers. To understand the state of migration, radioactivity of soil is separately measured for different depth ranges.
A chemical separation method that utilizes the nature of some metal ions being extracted from an aqueous solution to an organic solvent. It takes advantage of the fact that water (aqueous solution) does not mix with oil (organic solvent).

solvent extraction method
Equipment to measure radiation spectra. Commonly used spectrometers are α-ray spectrometers utilizing silicon semiconductor detectors and γ-ray spectrometers utilizing germanium semiconductor detectors.
It refers to distribution of radiation energy or radiation pulse height. Measuring α-ray spectra or γ-ray spectra allows us to determine the nuclide and radioactivity from the energy. Spectra measurement and analysis are collectively called spectrometry.
A radioactive isotope with a half-life of 28.8 years that is generated by nuclear fission of uranium and other nuclides. Its chemical nature is very close to calcium, and therefore it tends to be accumulated in bones when taken into our bodies. Strontium-90 that is found in nature came from atmospheric nuclear tests, but the current concentration of strontium-90 in rainwater and dust has dropped to about 1/20 of the concentration in the 1970s.
It is portable small radiation measurement equipment. Various survey meters, such as ionization chamber survey meters, GM tube survey meters, and NaI(Tl) scintillation survey meters are used depending on the type of radiation (e.g., α-ray, β-ray, γ-ray) to measure. Some survey meters for γ-rays can display not only count rates but also dose rates.

Ionization chamber type

GM tube type

NaI(Tl) scintillation type

ZnS(Ag) scintillation type
The system to swiftly predict the atmospheric concentration and exposure doses of radioactive materials near nuclear facilities and to provide information useful for planning and implementing evacuation in emergency cases of release of, or possible release of, a large amount of radioactive materials from a nuclear facility such as a nuclear power plant.
(Reference: Atomic Energy Encyclopedia ATOMICA)
T
Thorium is a naturally occurring radionuclide. The representative nuclide is thorium-232 (half-life: 14 billion years). Various nuclides that are generated starting with thorium-232 are called thorium-series nuclides (e.g., radium-224 and radon-220).
The abbreviation is TLD. When subjected to radiation, some materials form a state of atoms that can generate fluorescence. Heating such a material causes it to release faint light, which is detected to determine the dose of the radiation. TLD is used as radiation exposure dosimeter for radiation workers and as air radiation dosimeter in the environment.

Thermoluminescent dosimeter
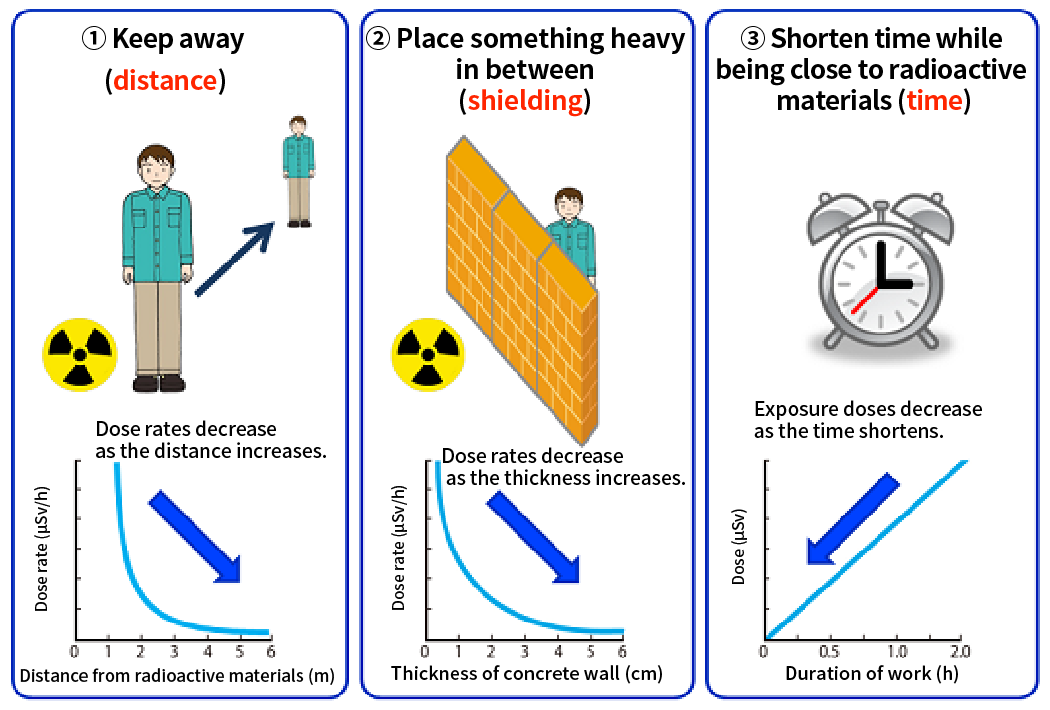
The Three Principles of Reduction of External Exposure are:
■ Distance
Keep the radiation source away from workers to reduce the air radiation dose rate during work
■ Shielding
Place a shielding object between the radiation source and workers to reduce exposure doses
■ Time
Shorten the time workers are exposed to radiation to reduce exposure doses
(Reference: Atomic Energy Encyclopedia ATOMICA) * Images are from the website of the Ministry of the Environment
A factor that is used as an indicator of the influence of radiation (radiosensitivity) that varies depending on the tissue or organ in our bodies.
(Reference: Atomic Energy Encyclopedia ATOMICA)
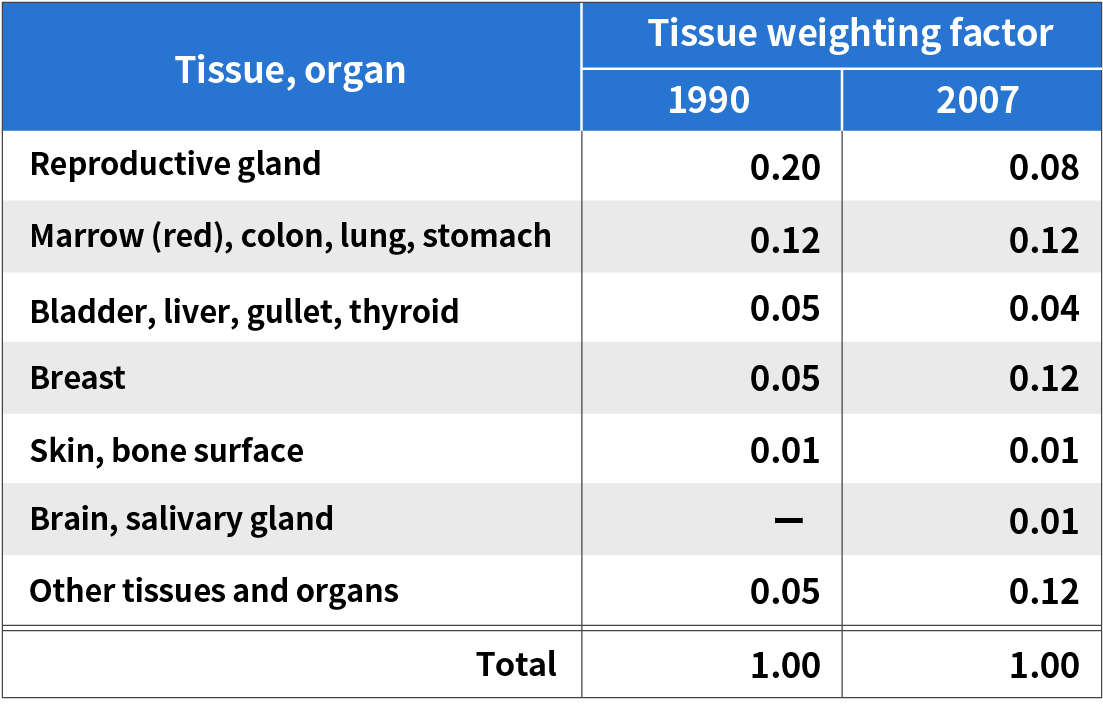
tissue weighting factor
Tritium, or hydrogen-3, is an isotope of hydrogen. It is expressed as 3H or T. It’s a radionuclide with a half-life of 12.3 years, which emits low-energy β-rays. In nature, tritium is generated by nuclear reaction between cosmic rays and atoms in the atmosphere.
U
According to JIS TS Z 0032:2012, uncertainty refers to “parameter, based on the information used, that characterizes the dispersion of the values that could reasonably be attributed to the measurand.”
It stands for the United Nations Scientific Committee on the Effects of Atomic Radiation that was launched as per the UN Recommendations in 1955.
Natural radionuclide that is contained in ores and soil. Its representative nuclides are uranium-238 (half-life: 4.5 billion years) and uranium-235 (half-life: 0.7 billion years). Uranium-238 decays with emitting α-rays and the decay produces further decay, resulting in a series of radionuclides. These nuclides are called uranium-series nuclides. Uranium-235 makes up only 0.7% of natural uranium, but is very important as a nuclide that undergoes fission by neutrons.
V
Vegetable of which the leaf part is consumed.
Vegetable of which the root part is consumed. For the purpose of this website, root vegetables include vegetables that grow underground, such as potatoes (modified stems) and onions (modified leaves), not limited to the “root” in the botanical definition.
