Environmental radioactivity and radiation are explained in text for easier understanding.
What is radiation? What is radioactivity?
Radiation includes alpha, beta, gamma, and neutron rays. Radioactivity refers to an ability to release such radiation.
Everything around us is made of atoms.
For example, the oxygen we breath in is made by two oxygen atoms binding to each other. An atom itself consists of a nucleus and negatively charged electrons that circle around the nucleus. A nucleus is made of positively charged protons and non-charged neutrons. The number of protons is the atomic number.
The atomic number of oxygen is eight. So, an oxygen nucleus has eight protons. In most cases, the number of neutrons in an oxygen atom is eight. However, some oxygen atoms have nine or 10 neutrons. Like this, nuclides with the same number of protons, atomic number, and different number of neutrons are called isotopes.
Nuclide means the type of nucleus that is determined by the number of protons and neutrons. The stability of a nucleus is governed by the balance in the number of protons and neutrons. Nuclides with the number of protons up to 20 are the most stable when the number of protons is the same as the number of neutrons. When the number of protons exceeds 20, the protons start to feel a stronger repulsive force to each other. Neutrons increase the attractive force between protons without increasing the repulsive force.
Therefore, when the number of protons becomes larger than 20, the number of neutrons starts to be greater than the number of protons (i.e., the ratio of the number of neutrons to the number of protons starts to be greater than one and keeps increasing), and by that the nucleus stays stable. When a nucleus is far larger, the repulsive force between protons is much stronger, and eventually the attractive force becomes unable to keep the protons together. At that point, the nucleus disintegrates, and releases a nucleus of helium (consisting of 2 protons and 2 neutrons).
For example, uranium-238 (consisting of 92 protons and 146 neutrons) releases one helium nucleus (2 protons and 2 neutrons) and become thorium-234 (90 protons and 144 neutrons). The helium nucleus released during such a decay is called an alpha particle. And such a decay is called alpha decay.
On the other hand, also when the ratio of the number of neutrons to the number of protons is too large, the nucleus becomes unstable.
For example, cesium-137 (55 protons and 82 neutrons), which was artificially created by atmospheric nuclear tests of uranium or plutonium, has a much larger number of neutrons compared with its stable cousin, cesium-133 (55 protons and 78 neutrons), and one neutron in the nucleus of cesium-137 releases an electron to become a proton. Since the number of protons in the nucleus increases by one, the nucleus changes to the element with the atomic number of 56, barium. This barium-137 (56 protons and 81 neutrons) is a stable nucleus. Such a decay is called beta decay. When cesium-137 decays to barium-137, in some cases cesium-137 directly becomes a stable barium-137 nucleus. In other cases, cesium-137 first becomes a semi-stable barium-137 nucleus (expressed as barium-137m to distinguish), and the barium-137m becomes barium-137. When barium-137m becomes barium-137, it emits electromagnetic waves with a very short wavelength.
Alpha particle, electron, and electromagnetic waves released by decay like in the examples above are called alpha, beta, and gamma rays, respectively, and they are collectively called radiation.
Radioactivity means ability to release such radiation.
Is it possible to measure radiation in the general environment?
Radiation can be measured using measurement equipment called a survey meter, even if the amount is very small.
We cannot sense radiation. We cannot see it, or hear it. But, measurement equipment allows us to measure it, even if the amount is very small. To measure radiation, equipment called a survey meter is used. Different types of survey meters are used depending on the type of radiation to measure.
Alpha rays are measured using ZnS(Ag) scintillation survey meters, beta rays are measured using GM tube survey meters, and gamma rays are measured using NaI(Tl) scintillation or ionization chamber survey meters.

ZnS(Ag) scintillation type

GM tube type

NaI(Tl) scintillation type

Ionization chamber type
Alpha rays can be stopped only by one sheet of paper, and beta rays cannot penetrate through a thin sheet of metal. Therefore, measurement of alpha and beta rays is limited to measurement of surface contaminations mostly.
Measurement of radiation in the air of general environment normally means gamma-ray measurement. The intensity of radiation is measured by air radiation dose rate, which is radiation dose per unit time. Air radiation dose rate is expressed either in μSv/h (microsievert per hour; micro means one millionth), which is 1 cm dose equivalent indicating the impact of radiation on the human body, or in μGy/h (microgray per hour), which is air kerma indicating the intensity of radiation in the air (the amount is essentially the same as air absorbed dose in the measurement for the general environment).
Air radiation dose rates in the general environment fluctuate by the region and the weather, because gamma-rays are emitted from naturally occurring radioactive materials such as uranium. Usually, air radiation dose rates are in the range of 0.02-0.10 μSv/h. At the beginning of rainfall or snowfall, air radiation dose rates can go up to 0.20 μSv/h.
NaI(Tl) scintillation survey meters are able to measure from 0.1 μSv/h up to around 30 μSv/h.
Ionization chamber survey meters are able to measure from 1 μSv/h up to roughly 10-300 mSv/h (millisievert per hour; milli means one thousandth).
Also, stationary monitoring posts are installed to continuously measure gamma-rays in the air. Detectors used in stationary monitoring posts are mostly NaI(Tl) and ionization chamber scintillation detectors, the same as survey meters. As a side note, the effective dose (μSv/h) can be estimated using air kerma (μGy/h), by simply multiplying air kerma by 0.8. However, in emergency situations, air kerma is to be regarded the same as effective dose to avoid confusion.

Stationary monitoring post
What are naturally occurring radioactive materials? What are artificial radioactive materials?
There are radioactive materials that exist in nature since the creation of the Earth and continue to emit radiation even now, and artificial radioactive materials created by mankind.
Naturally occurring radioactive materials
There are radioactive materials that have existed since the creation of the Earth and decay with emitting radiation even now. Such radioactive materials are called naturally occurring radioactive materials.
Typical naturally occurring radioactive materials are uranium-238, uranium-235, and thorium-232, as well as radium-226 and radon-222 generated from uranium-238 through radioactive decay. The reason why uranium-238, uranium-235, and thorium-232 exist on the Earth even now is because their half-life (time required for the number of atoms in a radioactive material to halve through decay) is 4.5, 0.07, and 14 billion years, respectively, which is as long as the age of the Earth, namely, 4.6 billion years.
Artificial radioactive materials
Separate from naturally occurring radioactive materials, there are radioactive materials created by mankind. Such radioactive materials are called artificial radioactive materials. For example, artificial radioactive materials strontium-90 and cesium-137 created by nuclear fission of uranium and plutonium in atmospheric nuclear tests conducted until 1980 still exist in the general environment.
Why do strontium-90 and cesium-137 created by atmospheric nuclear tests conducted until 1980 exist in the general environment even now?
Their half-life is relatively long and about half of them still exist in the general environment. However, doses of radiation from them are very small compared to the doses of natural or artificial radiation.
In atmospheric nuclear tests, uranium and plutonium underwent nuclear fission and created many artificial radioactive materials.
Among them, in particular, radioactive materials with mass numbers (sum of the number of protons and neutrons) near 90 and 140 were generated in abundance. The very nuclides corresponding to these mass numbers are strontium-90 (this number 90 refers to the mass number) and cesium-137.
The ratio of generation of fission products by nuclear fission of the parent nuclide is called fission yield.
The fission yield of strontium-90 or cesium-137 when uranium or plutonium undergoes nuclear fission is relatively high. For example, their fission yield is 5.9% and 6.2%, respectively, in the case of nuclear fission of uranium. In addition, the half-life of strontium-90 and cesium-137 is 29 and 30 years, respectively. This means, although several decades have passed since the last atmospheric nuclear test in 1980, about half of these radionuclides still remain.
Therefore, strontium-90 and cesium-137 are still found in the general environment, such as in the rainwater, soil, food and drinks.
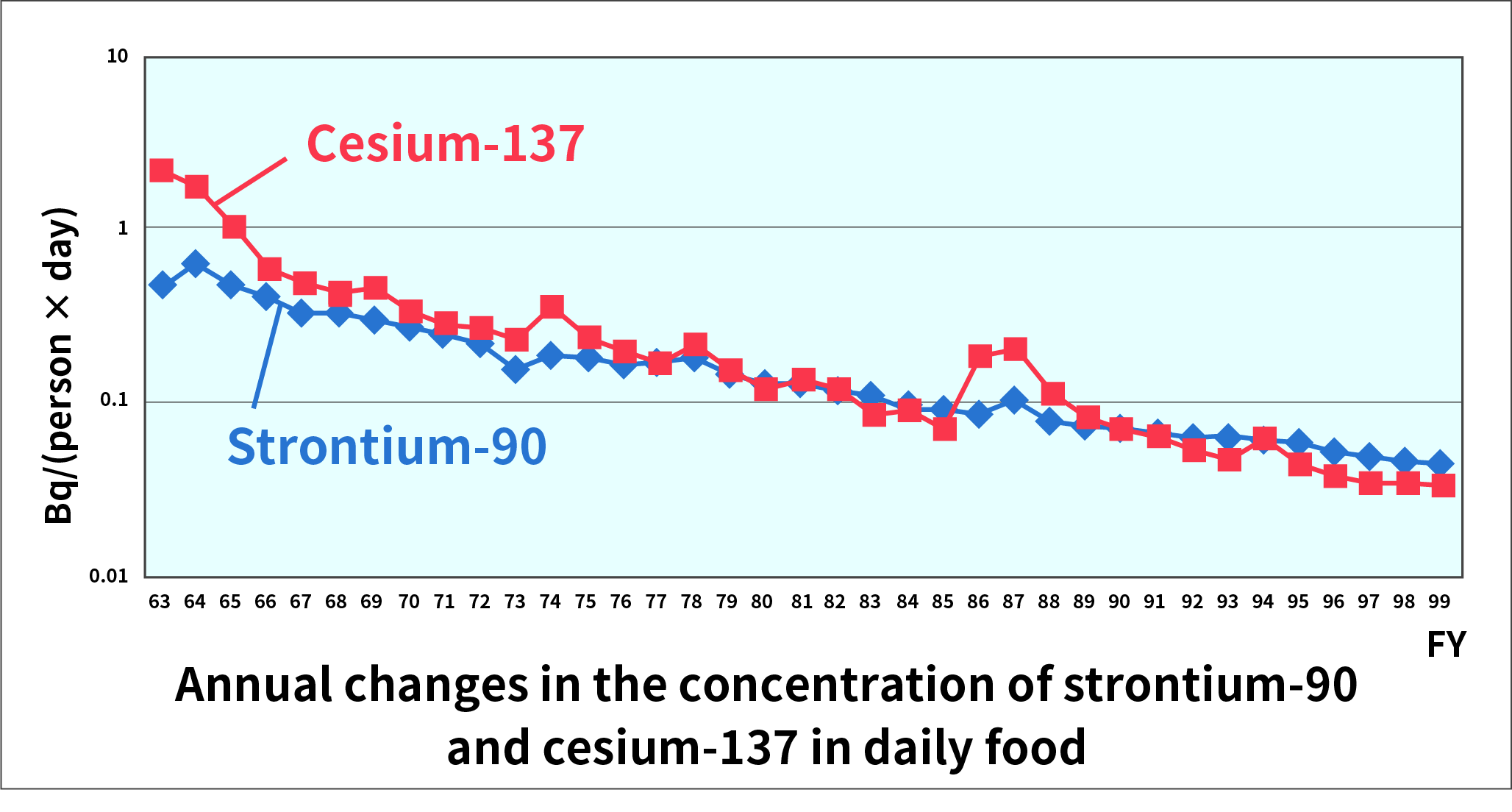
As an example, annual changes in the concentration of strontium-90 and cesium-137 in daily food (refers to three meals, breakfast, lunch and dinner we have daily) are shown in the figure below. Until 1980, the influence of atmospheric nuclear tests can be observed. Also, the impact of the accident at the Chernobyl Nuclear Power Plant that occurred in April 1986 was observed in FY1986 and FY1987. However, the doses of radiation from strontium-90 or cesium-137 are very small compared with doses of natural radiation or artificial radiation used for medical or other purposes.
How do you measure strontium-90 or cesium-137 in rainwater or daily food?
After reducing the volume of the sample (by evaporating the water component for rainwater and by incinerating for daily food), the sample is measured using measurement equipment.
Almost all radioactive materials emit gamma rays when they decay. A gamma ray has a single level of energy, and energy released from a material is specific to the material. Therefore, measuring gamma rays allows us to learn which radioactive materials are contained in rainwater or daily food by how much.
However, strontium-90 does not emit gamma rays at all when it undergoes radioactive decay. Instead, it emits beta rays. Different from gamma rays, beta rays have a continuous band of energy, and for that reason measuring beta rays doesn’t allow us to determine which radioactive material emitted the beta rays.
Therefore, after reducing the volume of the sample (by evaporating the water component for rainwater and by incinerating for daily food), strontium needs to be separated from the sample using reagents such as hydrochloric acid. Once strontium is separated, measuring beta rays of strontium-90 (in reality, since energy of beta rays from yttrium-90, a decay product of strontium-90, is larger than energy of beta rays from strontium-90, beta rays from yttrium-90 are measured) using a beta counter equipped with a GM tube allows us to determine how much strontium is contained.
Cesium-137 emits gamma rays and beta rays when it decays. After reducing the volume of rainwater or daily food, gamma rays emitted from cesium-137 (energy: 661.6 keV (kiloelectron volt)) are measured using a gamma counter equipped with a germanium semiconductor detector to determine how much cesium-137 is contained in the sample. Also, similar to strontium-90, when cesium is separated from the sample after reducing the volume and beta rays from the sample are measured, we can determine how much cesium is contained in the sample. Compared to the case of measuring gamma rays, this method allows for determining the content much more precisely. The content of radioactive material is expressed as radioactivity concentration.
The unit of radioactivity concentration is Bq/km2 for rainwater, and Bq/(person × day) for daily food. Bq (becquerel) is the number of nuclei that decay per second.

Beta counter equipped with GM tube
(low-background beta counter)


Gamma counter equipped with germanium semiconductor detector
Left: Seiko EG&G product
Right: Mirion Technologies Canberra product
I heard radon is a naturally occurring radioactive material. What is it?
Radon is a naturally occurring gaseous radioactive material, and disperses into the air from the ground and building materials.
Radon is a naturally occurring gaseous radioactive material, and disperses into the air from the ground and building materials. For that reason, radon sometimes accumulates in a closed building, but can be released to the outside by opening the windows.
When people say “radon,” they usually mean radon-222. Radon-222 is a radioactive material with a half-life of about 3.8 days, which decays with emitting alpha rays.
There are naturally occurring radioactive materials in addition to radon, and we receive radiation from them in our daily life. According to the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000 Report, the exposure dose we receive from naturally occurring radioactive materials is 2.4 mSv per year as the world average, and half of which is attributable to radon. Radon is taken into human bodies through breathing, and decays with emitting alpha rays.
Like people working in an underground radon mine, if a large quantity of radon is inhaled in an environment with a very high radon concentration, it is possible that materials created by radon after emitting radiation settle in the bronchi or lungs and radiation emitted from such materials causes lung cancer.
The International Commission on Radiological Protection (ICRP) has set forth the basic approach and action level of radiological protection against radon. According to that, as an action level of indoor radon concentration (radon level that requires taking some action), ICRP recommends 200-600 Bq/m3 (*1) (corresponding to an annual effective dose of 3-10 mSv). This recommendation is adopted in Europe and North America as laws and recommendations. The Report “About Exemption from Regulations on Natural Radioactive Materials” issued by the Radiation Council, Nuclear Regulation Authority in October 2003 states that “Radon in dwellings, etc., is excluded from the subjects of consideration in this Report since the action level is to be considered in the future as a target of intervention” and “Regarding radon, it is appropriate to consider the action level after investigation in general dwellings and workplaces have progressed.”
(*1) Bq/m3 (becquerel per cubic meter): unit of radioactivity of radon in a unit volume of air.
How do you measure radon?
Radon is measured using radon measurement equipment adopted in Europe and the US.
Surveys on the radon concentration have been conducted by national and local governments throughout the world since the 1980s, primarily in the European states and the US.
The results are provided in UNSCEAR reports and other materials, and the global average indoor radon concentration is reported to be 40 Bq/m3.
Also in Japan, surveys on the radon concentration have been conducted since 1985 targeting indoor, outdoor, and workplace environments. Radon is measured using radon measurement equipment adopted in Europe and the US. When radon in the air enters into measurement equipment placed at a measuring location and alpha rays released from the radon or its decay products hit a film placed inside, scratch marks are generated on the film. This film is chemically treated to make the scratch marks visible, and the number of the scratch marks are counted and converted to radioactivity concentration.
Passive radon–thoron discriminative measurement equipment

UFO type

Radosys product
What is the radon concentration in Japan?
The average radon concentration in dwellings in Japan is 15.5 Bq/m3, which is lower than the global average of 40 Bq/m3.
In Japan, radon concentration surveys started in 1985, and were conducted in dwellings, workplaces and outdoors up to 2002. The surveys saw the dwelling radon concentration in the range of 3.1-208 Bq/m3, with the average value of 15.5 Bq/m3. According to the UNSCEAR 2000 Report, the maximum and average global dwelling radon concentrations are 85,000 and 40 Bq/m3, respectively. The reason why the radon concentration in Japan is lower than the world average is considered to be because the structure and material (wood) of buildings encourage good ventilation.
On what and by whom are surveys on radioactivity in the general environment conducted?
Surveys on radioactivity in the general environment are conducted by the Secretariat of the Nuclear Regulation Authority with the cooperation of relevant ministries, agencies and prefectural governments.
Surveys on radioactivity in the general environment are conducted by the Secretariat of the Nuclear Regulation Authority with the cooperation of relevant ministries, agencies and prefectural governments.
With the cooperation of prefectures in Japan, the Secretariat of the Nuclear Regulation Authority conducts analysis of environmental samples, including rainwater, soil, white rice, vegetable, milk and daily food, for radionuclides such as strontium-90 and cesium-137.
Relevant ministries and agencies carry out surveys in the field of their expertise and authority.
For example, the Ministry of the Environment carries out surveys on air radiation dose rates on remote islands, the Ministry of Defense surveys high-altitude airborne dust, the Fisheries Agency surveys marine organisms, the Japan Meteorological Agency works on airborne dust and sea water, and the Japan Coast Guard surveys sea sediment.
Separate from them, as surveys pertaining to calling of nuclear powered warships of the US in the ports of Yokosuka, Sasebo, and Kin-Nakagusuku (in Okinawa), the Secretariat of the Nuclear Regulation Authority conducts measurements of air radiation dose rates and radiation count rates in sea water as well as collects and analyzes sea water and sea sediment at the time of departure, with the cooperation of the Fisheries Agency, Japan Coast Guard, Yokosuka City, Sasebo City, and Okinawa Prefecture.
The results of these surveys are available in the Environmental Radiation Database on this website. Using the data, you can create tables and charts like annual change graphs.
Look at the proceedings of environmental radioactivity survey results
Under what kind of system are emergency radioactivity surveys of nuclear facilities conducted?
Local governments conduct emergency monitoring, and the Secretariat of the Nuclear Regulation Authority and other relevant agencies provide support for the emergency monitoring.
If a nuclear facility in Japan enters into an emergency state, local governments are to conduct emergency monitoring and the Secretariat of the Nuclear Regulation Authority and other relevant agencies are to provide support for the emergency monitoring. In addition, if a nuclear-related accident occurs outside Japan, the Liaison Meetings for Radiological Countermeasures established under the Cabinet is to handle relevant surveys on the impact on Japan.
The publications below were used as reference in making these questions and answers.
(References)- Nuclear Emergency Response Guidelines: Nuclear Safety Commission
- Ordinary Times Monitoring (Supplementary to the Nuclear Emergency Response Guidelines): Radiation Monitoring Division, Radiation Monitoring Department, Nuclear Regulation Authority
- Emergency Monitoring (Supplementary to the Nuclear Emergency Response Guidelines): Radiation Monitoring Division, Radiation Monitoring Department, Nuclear Regulation Authority
- Nuclear Power Pocket Book: Japan Atomic Industrial Forum, Inc.
- Nuclear and Radiochemistry: Nobufusa Saito, et al. (transls.), Maruzen
- Concise Inorganic Chemistry: J.D. LEE (author), Hiroshi Hamaguchi (transl.), Tokyo Kagaku Dojin
- Measurement and Detection of Radiation: Nicholas Tsoulfanidis (author), Eiji Sakai (transl.), Gendai Kogaku Sha
- Radiation Data Book: Yukio Murakami, et al. (eds.), Chijin Shokan




