What is Committed Effective Dose?
Radioactive materials taken into the tissues of our bodies gradually decrease over time by natural decay and are released to the outside of the body through metabolism.
Committed effective dose is a radiation dose assuming that the dose that is to be received over a committed period of 50 years after intake is received in the first year, which is used as a standard for evaluating the long-term impact of internal exposure by food.
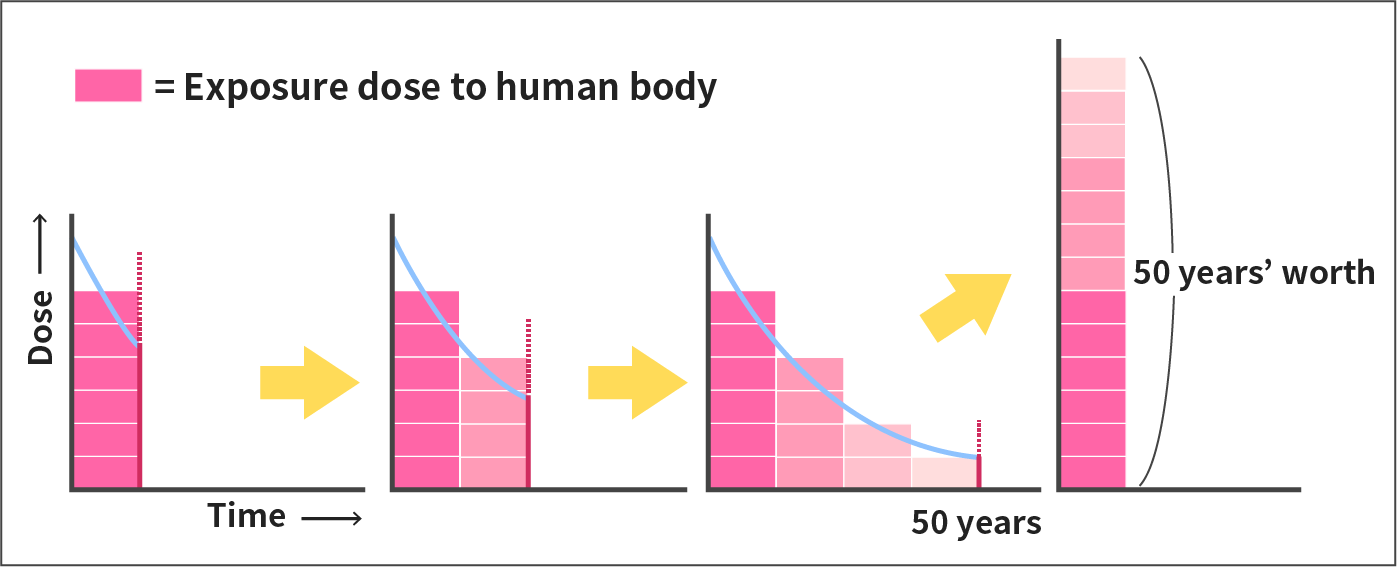
In the case of internal exposure where tissues and organs of our bodies are irradiated by radiation due to decay of radionuclides taken into the body, the dose to the tissues and organs changes with time. This temporal change in dose rate depends on the type, physical and chemical nature of the radionuclide, how the radionuclide was taken in, and the tissues or organs the nuclide is mostly taken into.
Usually, our bodies cannot selectively control the metabolism or the speed of excretion for radionuclides. Therefore, once a radionuclide is taken into our bodies, the effect (dose rate distribution and future cumulative dose) of the radionuclide in internal exposure is determined at the time of intake. The committed equivalent dose, H(τ,T), received by a tissue or organ, T, is expressed by the following formula:
H(τ,T)=∫h(t)dt
where the integration with respect to time is done from t0 to t0 + τ.
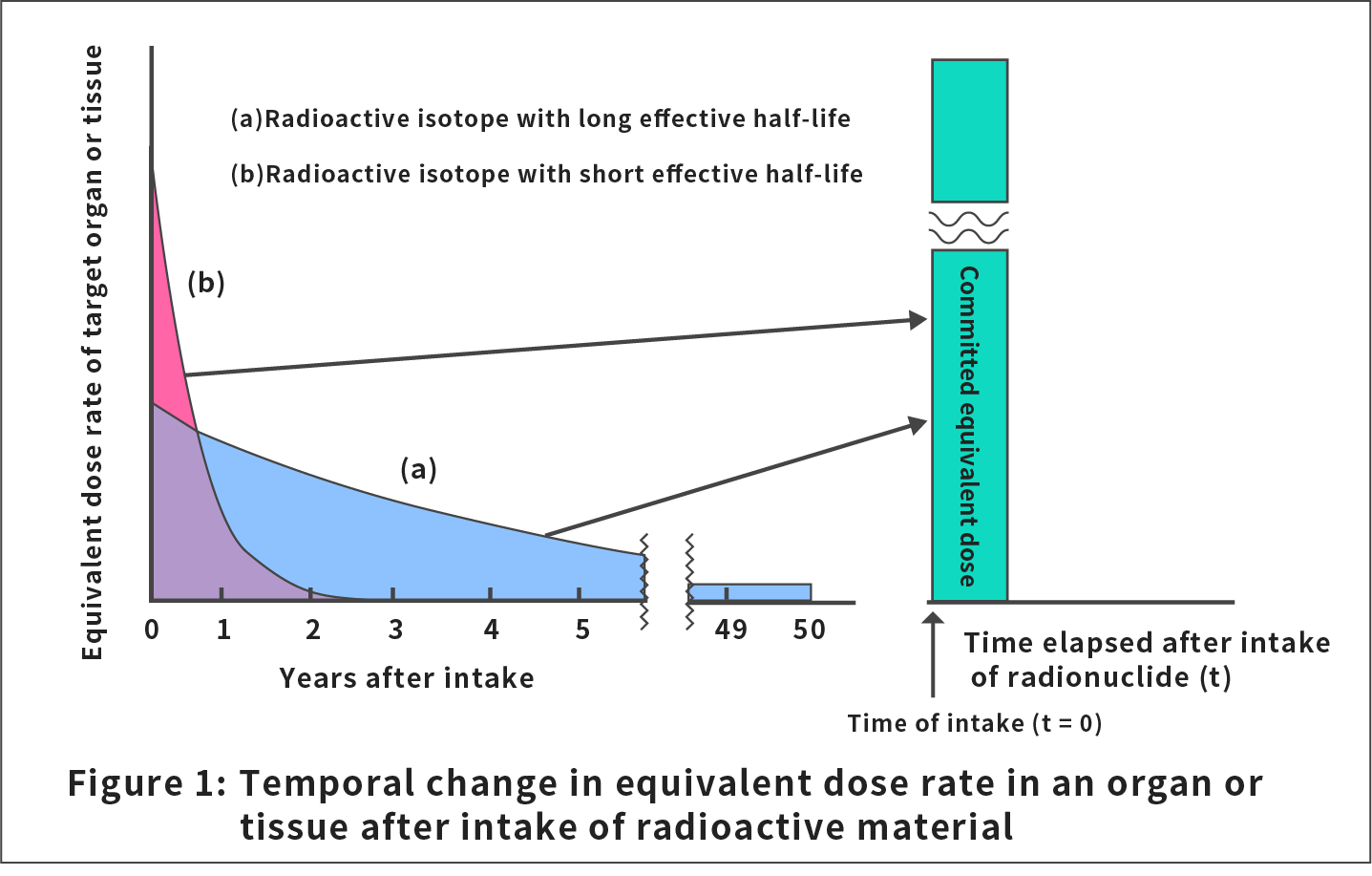
In the formula above, h(t) is the dose rate the tissue or organ, T, receives at time t, and the committed period, τ, is set to be 50 years for occupational exposure and for adults and 70 years for children and infants.
Figure 1 shows examples of h(t) in the formula above for radioactive materials with a long and short effective half-life (half-life that takes into account both the natural decay and discharge of radionuclides from the body) in a tissue or organ.
Committed effective dose, E(τ), is the sum of the products of the equivalent dose that a tissue or organ, T, receives from intake of radioactive materials and the appropriate tissue weighting factors, W(T), as shown in the following formula:
E(τ)=ΣW(T)・H(τ,T)
where the summation is done for all tissues and organs in the body.
However, if a radioactive material taken into our bodies adsorbs and remains in a tissue or organ, it is not easy to calculate the cumulative dose the tissue or organ receives. That is because calculation of the internal exposure dose requires measuring the amount of the radioactive material remaining in the tissues and organs and tracking the temporal change of the amount.
To simplify the calculation, in the case of internal exposure, the relationship between the amount of radioactive materials taken and the amount of dose a tissue or organ receives is predetermined. Using this relationship, the dose a human body receives is calculated based on the intake amount of radioactive materials.
The ratio of committed effective dose (mSv) of acute intake (or single dose intake) of 1 Bq of a radionuclide to 1 Bq of the radionuclide is called effective dose coefficient (mSv/Bq), which is used to calculate committed effective doses. Table 1 shows examples of effective dose coefficients.
Committed effective dose is calculated by the following formula, using effective dose coefficient:
[Committed effective dose (mSv)]=[Effective dose coefficient (mSv/Bq)]×[Nuclide intake per year (Bq)]×[Market dilution factor]×[Correction for reduction by cooking, etc.]
[Nuclide intake per year (Bq)]=[Annual average nuclide concentration in environmental samples]×[Annual intake of the food, drink, etc.]
* The market dilution factor and correction for reduction by cooking, etc., are applied where necessary.
In Japan, committed effective doses are added to effective doses of external exposure in the control of exposure of individuals by the Act on the Regulation of Nuclear Source Material, Nuclear Fuel Material and Reactors and other radiation control-related laws (so that the sum of the committed effective dose assuming received in one year after intake and the effective dose of external exposure for the year does not to exceed the annual dose limit).
Table 1 Examples of effective dose coefficients for converting intake of radionuclide to internal exposure dose
| Column 1 | Column 2 | Column 3 | |
|---|---|---|---|
| Type of radioactive material | Effective dose coefficient for inhalation (mSv/Bq) |
Effective dose coefficient for ingestion (mSv/Bq) |
|
| Nuclide | Chemical form, etc. | ||
| 3H | Water | 1.8×10-8 | 1.8×10-8 |
| 60Co | Compounds other than oxides, hydroxides, and inorganic compounds (ingestion) | 3.4×10-6 | |
| 60Co | Oxides, hydroxides, and inorganic compounds (ingestion) | 2.5×10-6 | |
| 60Co | Compounds other than oxides, hydroxides, halides, and nitrates | 7.1×10-6 | |
| 60Co | Oxides, hydroxides, halides, and nitrates | 1.7×10-5 | |
| 90Sr | Compounds other than strontium titanate | 3.0×10-5 | 2.8×10-5 |
| 90Sr | Strontium titanate | 7.7×10-5 | 2.7×10-6 |
| 131I | Steam | 2.0×10-5 | |
| 131I | Methyl iodide | 1.5×10-5 | |
| 131I | Compounds other than methyl iodide | 1.1×10-5 | 2.2×10-5 |
| 137Cs | All compounds | 6.7×10-6 | 1.3×10-5 |
| 239Pu | Compounds other than nitrates and insoluble oxides (ingestion) | 2.5×10-4 | |
| 239Pu | Nitrates (ingestion) | 5.3×10-5 | |
| 239Pu | Insoluble oxides (ingestion) | 9.0×10-6 | |
| 239Pu | Compounds other than insoluble oxides | 3.2×10-2 | |
| 239Pu | Insoluble oxides | 8.3×10-3 | |
From Japan Radioisotope Association (ed.): Public Notice of the Science and Technology Agency, No. 5, October 23, 2000 (Specifying Standards for the Quantities, etc. of Radiation-Emitting Isotopes) Appended Table 2. Isotope Statute Book (I) 2005 Edition (October 2005), p375
References
- Japan Radioisotope Association (ed.): ICRP Publ. 42, A Compilation of the Major Concepts and Quantities in Use by ICRP, Maruzen (June 1986)
- Japan Radioisotope Association (transl.): ICRP Publ. 60, 1990 Recommendations of the International Commission on Radiological Protection, Maruzen (July 1991)
- Guidance for Environmental Radiation Monitoring (Nuclear Safety Commission, partially amended in March 2001)
- Glossary of Basic Terms in Nuclear Emergency Prevention
- Atomic Energy Encyclopedia ATOMICA http://www.rist.or.jp/atomica/index.html*Japanese
Committed Effective Dose Calculation Formula
Committed effective dose is calculated using the formula below(*1).
Committed effective dose H (mSv) = 0.001×m×d×p×a×f1×f2
m: Intake of food and drink (g/day)
The values of the intake of nutrition, etc., by food group (nationwide) in the National Health and Nutrition Survey 2005 conducted by the Ministry of Health, Labour and Welfare will be used. The values are provided in a table summarizing the intake (g) per person per day, arranged by food group.
d: Number of days of intake (days)
It will be 365 days, since we are looking to obtain dose per year.
p: Effective dose coefficient (mSv/Bq)
Dose coefficients for ingestion are specified in ICRP Publ. 72 for each nuclide, which will be used.
Example: 2.8 × 10-5 for Sr-90, 1.3 × 10-5 for Cs-137
a: Radioactivity concentration (Bq/kg)
Data of radioactivity analysis for food samples collected in prefectures summarized by nuclide will be used.
f1: Market dilution factor
f2:: Correction for reduction by cooking, etc.
These values vary depending on the conditions, such as the distribution channel and cooking method. Here, we use the value of 1 as the most severe condition.
(*1) Guidance for Environmental Radiation Monitoring (Nuclear Safety Commission, partially amended in March 2001)
Committed Effective Dose Calculation Example
As an example, the committed effective dose is calculated below for Japanese jack mackerel.
- The concentration of radionuclide Cs-137 in Japanese jack mackerel is to be 0.20 Bq/kg (from the result of search on the Radioactivity in Food page).
- The intake of Japanese jack mackerel is 12.5 g per person per day (from the list of the intake of nutrition, etc., by food group in the National Health and Nutrition Survey 2005, Ministry of Health, Labour and Welfare). This is multiplied by 365 to obtain the intake per year.
- The effective dose coefficient of Cs-137 is 1.3 × 10-5 (from ICRP Publ. 72).
- Both the market dilution factor and correction for reduction by cooking, etc., are to be 1 (as the most severe condition that ignores market dilutions and correction for reduction by cooking, etc.).
These values are inserted into the committed effective dose calculation formula.
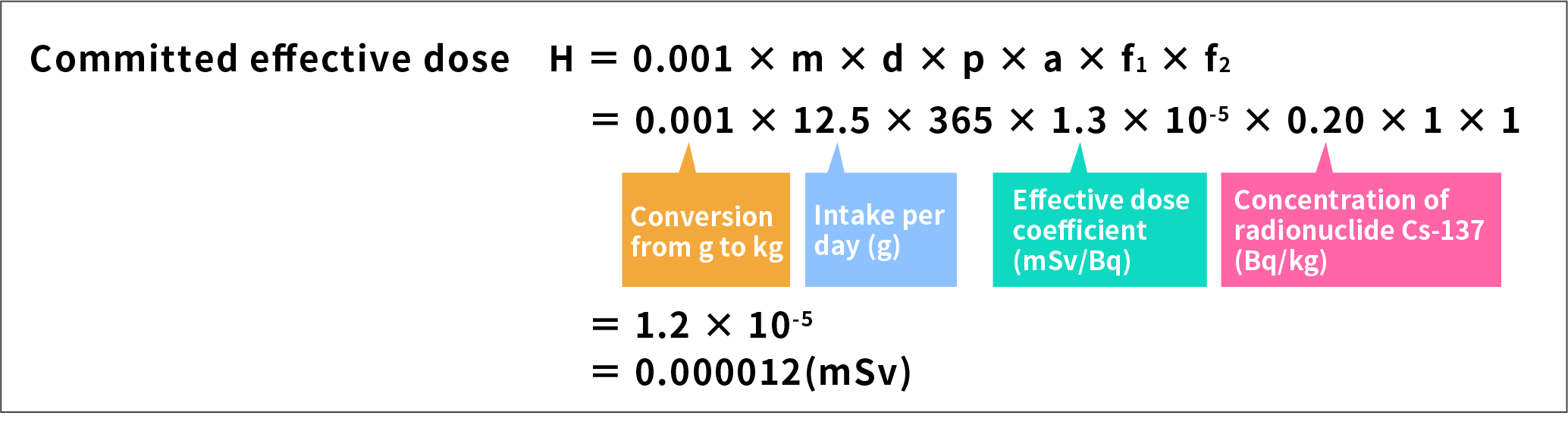
From this calculation, the committed effective dose of Cs-137 in the case of Japanese jack mackerel is 0.000012 mSv per year.
If data for other radionuclides is available for the same food, the committed effective dose is calculated for these radionuclides and added up.
Note that this calculation result simply shows the committed effective dose for the typical model case; it does not mean an assessment of exposure dose for certain individuals.
Presuppositions of Committed Effective Dose Calculation
In this website, the presuppositions below are adopted in the calculation of committed effective dose using this formula.
- Average nuclide concentration in food and drink
The results of food radioactivity level surveys and environmental radioactivity level surveys conducted from FY1989 to FY2005 are used.
However, in the case of calculating committed effective doses using the data searched on the Radioactivity in Food page, the survey period becomes longer than this since all relevant data are included in the search. - Annual intake of food and drink
The values of the intake of nutrition, etc., by food group in the National Health and Nutrition Survey 2005, Ministry of Health, Labour and Welfare are used. - Calculation target radionuclides
Calculations are made for artificial radionuclides Sr-90, Cs-137, and Pu-239+240 and natural radionuclides Pb-210, Po-210, Ra-226, Th-232, and U-238 that have a relatively long half-life and still remain in the environment.
Regarding natural radionuclide K-40 that exists in our bodies, see “About potassium 40 (K-40)” below. - Radioactivity measurement values below the limit of detection
Data below the limit of detection were excluded from calculation of committed effective doses, because such data are indicated as “Not detected” or “ND” and cannot be handled as numerical values.
About potassium 40 (K-40)
Potassium is an essential element for plants and animals. It widely exists in our bodies and in nature. The large majority of potassium is stable and does not emit radiation. However, a very small portion of potassium (*2) is K-40 that emits radiation (*3).
When potassium is taken into our bodies through food and drinks, the amount of potassium increases. However, our bodies release the same amount and therefore potassium is always maintained at a constant level.
Natural radionuclide K-40 exists in our bodies at a radioactivity of about 4000 becquerels (Bq). K-40 taken into our bodies through food and drinks is about 50 Bq per day, and the same amount is released by our body’s mechanism to discharge an excess amount of potassium. The annual exposure dose by K-40 is 0.17 millisieverts (mSv).
(*2) K-40 makes up 0.0117% (approx. 1 in 10,000) of the total amount of potassium.
(*3) K-40 has a half-life of 1.28 billion years, and releases beta- and gamma-rays.
